COVID-19 VACCINATIONS - SPUTNIK V IN INDIA AND OUTSIDE OF INDIA
I want to thank all of you(my visual audience) for the reactions,feedbacks,enthusim towards my 1st ever blog on Covid-19 vaccination in India.So, this is my 2nd blog post and today I will try to give you information about Covid-19 vaccination outside India as I promised.
Depending on the country, Covid-19 vaccination campaigns started in December 2020. Since the available quantities of vaccine are not sufficient to meet the needs of the population at once, people at risk and those directly involved in the fight against the pandemic are the first to be vaccinated.
The table below ranks countries according to the total number of doses administered as of 27 August 2021,Worldwide.
In the absence of a global database, the statistics are based on publications from country-specific health agencies and ministries.
| Rank | Country | Doses * | Rank | Country | Doses * | Rank | Country | Doses * |
|---|---|---|---|---|---|---|---|---|
| 1 | China | 1.99 billion | 11 | Mexico | 81.92 million | 21 | Saudi Arabia | 35.17 million |
| 2 | India | 597.92 million | 12 | Russia | 78.05 million | 22 | Colombia | 33.70 million |
| 3 | United States | 364.84 million | 13 | Italy | 76.21 million | 23 | Malaysia | 32.64 million |
| 4 | Brazil | 181.91 million | 14 | England | 75.24 million | 24 | Philippines | 31.43 million |
| 5 | Japan | 122.22 million | 15 | Spain | 64.31 million | 25 | Morocco | 31.25 million |
| 6 | Germany | 100.19 million | 16 | Canada | 52.73 million | 26 | Chile | 28.20 million |
| 7 | Indonesia | 92.11 million | 17 | Pakistan | 47.80 million | 27 | Thailand | 25.82 million |
| 8 | Turkey | 91.08 million | 18 | Argentina | 39.95 million | 28 | Bangladesh | 24.25 million |
| 9 | United Kingdom | 89.87 million | 19 | South Korea | 39.29 million | 29 | Netherlands | 21.82 million |
| 10 | France | 84.99 million | 20 | Poland | 35.92 million | 30 | Iran | 19.89 million |
| Rank | Country | Doses * | Rank | Country | Doses * | Rank | Country | Doses * |
|---|---|---|---|---|---|---|---|---|
| 31 | Sri Lanka | 18.10 million | 41 | Cuba | 12.70 million | 51 | Austria | 10.36 million |
| 32 | Cambodia | 17.95 million | 42 | Sweden | 12.22 million | 52 | Romania | 9.69 million |
| 33 | United Arab Emirates | 17.84 million | 43 | Uzbekistan | 12.10 million | 53 | Switzerland | 9.45 million |
| 34 | Australia | 17.75 million | 44 | Kazakhstan | 11.57 million | 54 | Nepal | 8.87 million |
| 35 | Vietnam | 17.65 million | 45 | Czechia | 11.34 million | 55 | Singapore | 8.78 million |
| 36 | Peru | 17.50 million | 46 | Greece | 11.25 million | 56 | Ukraine | 8.46 million |
| 37 | Ecuador | 17.07 million | 47 | South Africa | 11.08 million | 57 | Denmark | 8.43 million |
| 38 | Belgium | 15.94 million | 48 | Dominican Republic | 10.93 million | 58 | Egypt | 8.00 million |
| 39 | Portugal | 14.35 million | 49 | Hungary | 10.56 million | 59 | Scotland | 7.69 million |
| 40 | Israel | 13.11 million | 50 | Taiwan | 10.50 million | 60 | Hong Kong | 7.23 million |
| Rank | Country | Doses * | Rank | Country | Doses * | Rank | Country | Doses * |
|---|---|---|---|---|---|---|---|---|
| 61 | Ireland | 6.68 million | 71 | Tunisia | 5.05 million | 81 | Zimbabwe | 3.96 million |
| 62 | Finland | 6.59 million | 72 | Venezuela | 4.68 million | 82 | Paraguay | 3.78 million |
| 63 | Norway | 6.42 million | 73 | Slovakia | 4.49 million | 83 | Oman | 3.38 million |
| 64 | Azerbaijan | 6.34 million | 74 | Qatar | 4.31 million | 84 | Croatia | 3.23 million |
| 65 | Myanmar | 6.25 million | 75 | Mongolia | 4.31 million | 85 | Lithuania | 3.10 million |
| 66 | Jordan | 6.22 million | 76 | Algeria | 4.15 million | 86 | Honduras | 3.05 million |
| 67 | El Salvador | 5.90 million | 77 | Costa Rica | 4.03 million | 87 | New Zealand | 2.93 million |
| 68 | Serbia | 5.75 million | 78 | Panama | 4.03 million | 88 | Belarus | 2.68 million |
| 69 | Bolivia | 5.48 million | 79 | Guatemala | 4.03 million | 89 | Kenya | 2.50 million |
| 70 | Uruguay | 5.38 million | 80 | Nigeria | 3.97 million | 90 | Bahrain | 2.47 million |
| Rank | Country | Doses * | Rank | Country | Doses * | Rank | Country | Doses * |
|---|---|---|---|---|---|---|---|---|
| 91 | Kuwait | 2.38 million | 101 | Afghanistan | 1.81 million | 111 | Estonia | 1.24 million |
| 92 | Lebanon | 2.36 million | 102 | Rwanda | 1.72 million | 112 | North Macedonia | 1.18 million |
| 93 | Ethiopia | 2.35 million | 103 | Senegal | 1.71 million | 113 | Palestine | 1.08 million |
| 94 | Bulgaria | 2.26 million | 104 | Mauritius | 1.50 million | 114 | Cyprus | 1.07 million |
| 95 | Iraq | 2.10 million | 105 | Latvia | 1.48 million | 115 | Georgia | 1.07 million |
| 96 | Laos | 2.05 million | 106 | Albania | 1.39 million | 116 | Kyrgyzstan | 1.06 million |
| 97 | Tajikistan | 1.97 million | 107 | Uganda | 1.30 million | 117 | Guinea | 1.06 million |
| 98 | Mozambique | 1.96 million | 108 | Cote d'Ivoire | 1.29 million | 118 | Bhutan | 1.04 million |
| 99 | Slovenia | 1.87 million | 109 | Ghana | 1.27 million | 119 | Libya | 1.02 million |
| 100 | Angola | 1.84 million | 110 | Moldova | 1.25 million | 120 | Bosnia and Herzegovina | 928 126 |
| Rank | Country | Doses * | Rank | Country | Doses * | Rank | Country | Doses * |
|---|---|---|---|---|---|---|---|---|
| 121 | Trinidad and Tobago | 871 998 | 131 | Zambia | 558 307 | 141 | Equatorial Guinea | 336 579 |
| 122 | Malawi | 840 736 | 132 | Togo | 535 518 | 142 | Gambia | 321 385 |
| 123 | Sudan | 829 682 | 133 | Iceland | 522 977 | 143 | Suriname | 314 963 |
| 124 | Malta | 793 015 | 134 | Jamaica | 496 976 | 144 | Yemen | 311 483 |
| 125 | Fiji | 778 159 | 135 | Niger | 490 549 | 145 | Namibia | 305 086 |
| 126 | Luxembourg | 756 276 | 136 | Guyana | 455 209 | 146 | Congo | 290 287 |
| 127 | Maldives | 676 694 | 137 | Botswana | 434 644 | 147 | Somalia | 282 522 |
| 128 | Nicaragua | 612 799 | 138 | Cameroon | 418 13 | 148 | Brunei | 267 132 |
| 129 | Kosovo | 601 701 | 139 | Syria | 388 52 | 149 | Cape Verde | 262 164 |
| 130 | Macao | 582 592 | 140 | Montenegro | 380 803 | 150 | Mali | 259 719 |
| Rank | Country | Doses * | Rank | Country | Doses * | Rank | Country | Doses * |
|---|---|---|---|---|---|---|---|---|
| 151 | Mauritania | 254 155 | 161 | Jersey | 146 855 | 171 | Central African Rep | 95 862 |
| 152 | Eswatini | 239 519 | 162 | New Caledonia | 144 974 | 172 | DR Congo | 87 91 |
| 153 | Armenia | 239 342 | 163 | Seychelles | 143 49 | 173 | Bermuda | 84 022 |
| 154 | Comoros | 239 158 | 164 | Gabon | 130 798 | 174 | Burkina Faso | 71 51 |
| 155 | Sierra Leone | 225 38 | 165 | Bahamas | 129 017 | 175 | Djibouti | 58 245 |
| 156 | Tanzania | 218 621 | 166 | Samoa | 124 96 | 176 | South Sudan | 56 989 |
| 157 | Belize | 217 385 | 167 | Benin | 117 984 | 177 | Chad | 45 39 |
| 158 | Madagascar | 197 001 | 168 | Liberia | 113 68 | 178 | Monaco | 44 06 |
| 159 | Barbados | 195 008 | 169 | Papua New Guinea | 113 052 | 179 | Liechtenstein | 42 574 |
| 160 | Curacao | 181 226 | 170 | Lesotho | 103 778 | World | 5.08 billion | |
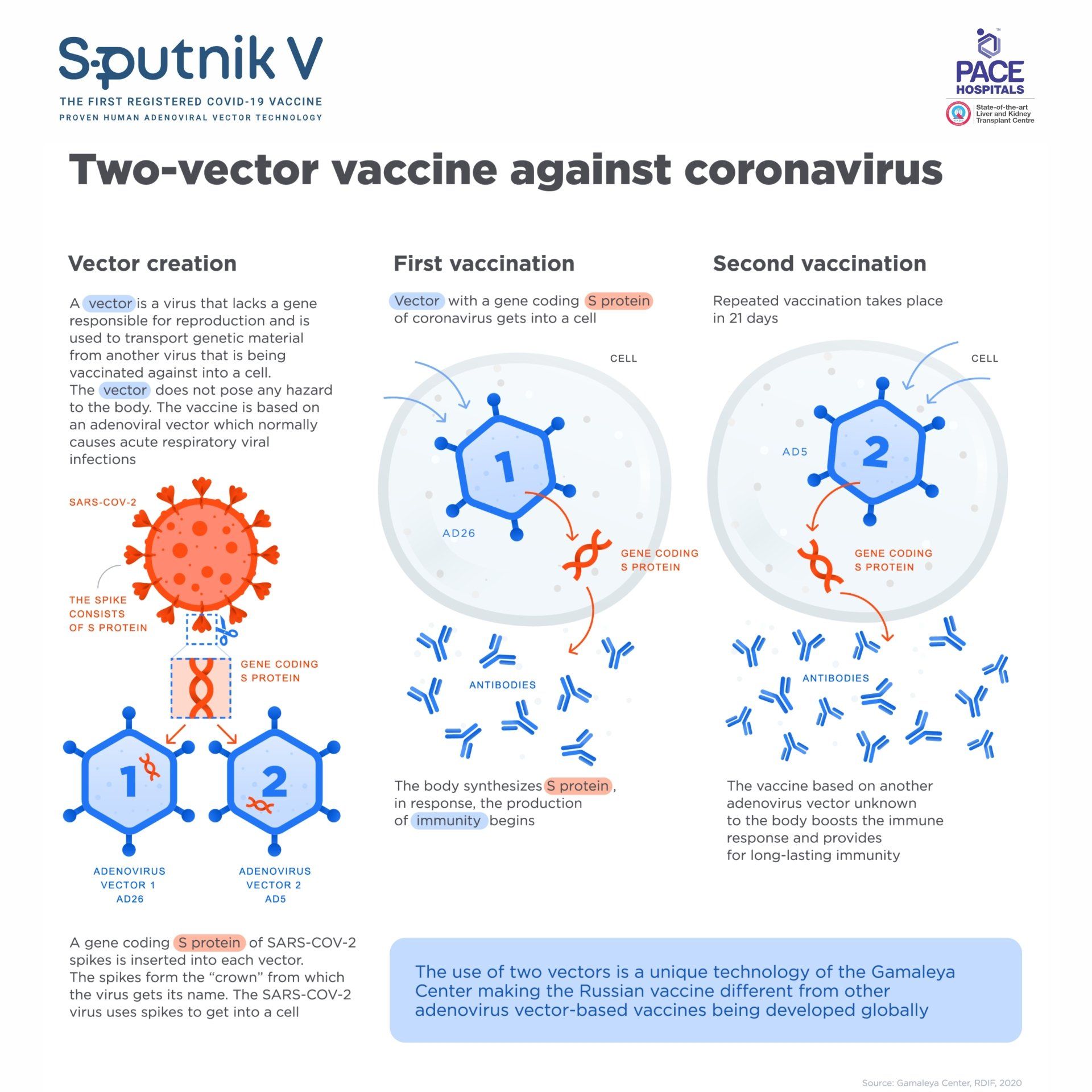
SPUTNIK V : Sputnik V, developed by the Gamaleya National Center of Epidemiology and Microbiology,Russia.The Russian COVID-19 vaccine Sputnik V (Gam-COVID-Vac) is an adenoviral-based, two-part whole viral vector virus vaccine against the SARS-CoV-2 coronavirus. Initially produced in Russia, Sputnik V uses a weakened virus to deliver small parts of a pathogen and stimulate an immune response.The Sputnik V (Gam-COVID-Vac) vaccine reduces the time taken for the actual development of immunity to SARS-CoV-2, the virus behind the COVID-19 pandemic.It is a vector vaccine based on adenovirus DNA, in which the SARS-CoV-2 coronavirus gene is integrated. Adenovirus is used as a “container” to deliver the coronavirus gene to cells and start synthesizing the new coronavirus's envelope proteins, “introducing” the immune system to a potential enemy.
The Sputnik V (Gam-COVID-Vac) is based on safe and effective human adenovirus vector platform using two different adenoviral vectors - Adenovirus 26 (Ad26) and Adenovirus 5 (Ad5) as an expression of SARS-CoV-2 spike protein gene.
The use of two varying serotypes is a unique approach that provide long-lasting immunity and allows to boost the immune response. The carrier viruses are modified and cannot begin a productive infection; they enter cells, express the spike protein, and then stop. High sensitivity recorded that a few adenovirus genes were expressed, although at very low level.
Eventually, the vaccine-infected cells are destroyed by the very immunity they are designed to evoke. Recombinant adenoviruses (rAD) have been used widely as vaccine vectors due to their quality to accommodate large genetic payloads and, although unable to replicate, they trigger the intuitive immunity sensors sufficiently to make sure a robust immune system in nature.
A heterologous recombinant adenovirus approach is shared with the chimpanzee adenovirus (ChAdOx) vectored COVID-19 vaccine by Oxford/AstraZeneca, Adenovirus 26 (Ad26) vectored COVID-19 vaccine by the Johnson & Johnson, and the Adenovirus 5 (Ad5) vectored COVID-19 vaccine by CanSinoBIO-Beijing Institute of Biotechnology.
Now
Q)1) HOW ADENOVIRAL VECTOR-BASED VACCINES WORK
“Vectors” are vehicles, which can induce a genetic material from another virus into a cell. The gene from adenovirus, which causes the infection, is removed while a gene with the code of a protein from another virus spike is inserted. This inserted element is safe for the body but still helps the immune system to react and produce antibodies, which protect us from the infection.
The technological platform of adenovirus-based vectors makes it easier and faster to create new vaccines through modifying the initial carrier vector with genetic material from new emerging viruses that helps to create new vaccines in relatively short time. Such vaccines provoke a strong response from a human immune system.
Human adenoviruses are considered as some of the easiest to engineer in this way and therefore they have become very popular as vectors.
Q)2) SAFETY AND EFFICACY
After the start of the COVID-19 pandemic Russian researchers extracted a fragment of genetic material from novel coronavirus SARS-COV-2, which codes information about the structure of the spike S-protein, which forms the virus’ “crown” and is responsible for connection with human cells. They inserted it into a familiar adenovirus vector for delivery into a human cell creating the world’s first COVID-19 vaccine.
In order to ensure lasting immunity Russian scientists came up with a breakthrough idea to use two different types of adenovirus vectors (rAd26 and rAd5) for the first and second vaccination, boosting the effect of the vaccine.
The use of human adenoviruses as vectors is safe because these viruses, which cause the common cold, are not novel and have been around for thousands of years.
Efficacy of Sputnik V against COVID-19 was reported at 91.6%. The figure is based on the analysis of data on 19,866 volunteers, who received both the first and second doses of the Sputnik V vaccine or placebo at the final control point of 78 confirmed COVID-19 cases.
Q)3) EFFICACY AGAINST NEW STRAINS
On 12.07.2021, a study on the efficacy of Sputnik V against new strains of coronavirus was published in the leading international magazine Vaccines by the Gamaleya Research Institute for Epidemiology and Microbiology.
The vaccine produces protective neutralising antibody titres against new strains including Alpha B.1.1.7 (first identified in the UK), Beta B.1.351 (first identified in South Africa), Gamma P.1 (first identified in Brazil), Delta B.1.617.2 and B.1.617.3 (first identified in India) and variants B.1.1.141 and B.1.1.317 with mutations in the receptor-binding domain (RBD) identified in Moscow.
The study methodology was based on assessing the viral neutralising activity (VNA) using live virus, which provides the most reliable data and is the accepted standard. The study compared the VNA of human serum after vaccination with Sputnik V on global strain samples with the VNA against the original strain B.1.1.1. Serum was sampled from individuals immunised with both components of Sputnik V.
The data obtained demonstrate that Sputnik V retains its protective properties against new strains. The reduction in viral neutralising activity of Sputnik V against a number of strains was significantly lower compared to data published by manufacturers of other vaccines that earlier confirmed their efficacy against new coronavirus mutations.
Q)4)When will mass production of the Russian vaccine start?
In 2020, mass production of Sputnik V was launched in Russia and abroad. So far, more than 14 countries have announced the vaccine production launch, including India, China, Brazil, Mexico, Egypt, Iran, Italy, South Korea, Argentina, Kazakhstan, the Republic of Belarus, Serbia, Turkey, Vietnam, etc.
Sputnik V has been approved for use in 70 countries with a total population of 4 billion people. The list of countries includes the Americas, the Middle East, Europe, Asia and Africa. The list is expected to expand. Vaccination with Sputnik V is underway in more than 50 countries on 4 continents, including Argentina, Hungary, Bolivia, Algeria, Montenegro, Paraguay, etc.
Q)5)When will Russia provide full scientific data behind the vaccine?
The vaccine Phase I–II and Phase III clinical trials analysis was published in The Lancet , one of the leading international medical magazines.
Q)6)When will the vaccine be available?
Sputnik V is available for the population of Russia and other countries where the regulatory authority has approved the vaccine application within the national anti-COVID-19 mass vaccination program. Russia has launched Sputnik V serial production at different in-country facilities, primarily for domestic use. Our international partners are currently manufacturing Sputnik V for foreign use. Subscribe to Sputnik V social media feeds to see Sputnik V international production and distribution data.
Q)7)How to obtain/purchase the vaccine?
Sputnik V will be allowed for use by a certain country’s population after being approved by the respective regulatory authority within the national anti-COVID-19 mass vaccination program. Subscribe to Sputnik V social media feeds to see Sputnik V international production and distribution data.
Q)8)What will be the price of Sputnik V vaccine?
Production costs and logistics will be the factors affecting the price for specific countries. In many countries, e. g. Russia, national medical insurance programs will cover the vaccination costs. As we are not aiming at extra high profits from the vaccine sales, the price will be competitive, max $10/dose,Rs 800/dose in India.
Q)9)How to get Sputnik V Vaccine in India?
In April 2021, Drugs Controller General of India (DCGI) has been given approval for restricted emergency use of "The Sputnik V vaccine" in INDIA.
On 14 May 2021 Russia’s Sputnik-V vaccine also called as Gam-COVID-Vac, a two combined vector vaccine, has been launched in INDIA by Dr Reddy’s Laboratories Limited (DRL), Hyderabad-based pharmaceutical company.
G V Prasad, managing director of Dr Reddy’s Laboratories said - “COVID-19 vaccination is most effective tool to fight against coronavirus, everyone should come forward and take vaccine."
We Pace Hospitals have started administering the Sputnik V Vaccine at our Hitech City Branch located near Hitech City Metro Station, Hyderabad, Telangana, India and at Sarath City Capital Mall, Gachibowli - Miyapur Rd, Whitefields, Hitech City, Kondapur, Hyderabad, Telangana under #LargestVaccineDrive initiative.
Vaccine Centres will provide onsite registration facilities, people can walk-in directly to vaccination centres and get vaccinated or else book Sputnik V Vaccine slot through online CoWin registration in INDIA.
Q)10)Serum Institute of India - the Sputnik V COVID-19 vaccine
Serum Institute of India Pvt. Ltd., world's largest vaccine manufacturer by number of doses produced and sold globally will start manufacturing the Sputnik V Vaccine in India, first batch will start rolling from September 2021. RDIF mentioned, around 30 crore doses will be produced in INDIA per year.
The Drug Controller General of India (DCGI) gave approval to import cell and vector samples from Russia's The Gamaleya National Center of Epidemiology and Microbiology.
Kirill Dmitriev, CEO of RDIF said: "Strategic partnership with Russian Direct Investment Fund (RDIF) and Serum Institute of India (SII) will help in scaling up the production with high efficacy and excellent safety profile. Globally, this will help in battling with COVID-19 and saving lives."
Mr. Adar Poonawalla (CEO), SII said "High efficacy and excellent safety profile, the Sputnik V Vaccine is available for the people across the globe and strengthen people to fight against SARS-CoV-2."
Q)11)What are the side effects of Sputnik V Vaccine?
- A)Pain, redness, or swelling at the site of injection
- B)Asthenia (lack of energy / abnormal physical weakness)
- C)Fatigue (feeling tired)
- D)Body and muscle pain
- E)Cough and Sore throat
- F)Runny nose
- G)Fever and Chills
- H)Nausea and Vomiting
- I)Diarrhea
- J)Headache
The Sputnik V Vaccine recorded excellent safety results and proven effective against new strains of coronavirus. 100% people showed cellular immune response against the S Protein of SARS-CoV-2.
Usually side effects go away within 24 to 48 hrs, if any side effects persist for longer than 48 hrs contact the vaccination centre or your physician and start medications to overcome side effects.
There was zero evidence of serious adverse events and anaphylactic shock after vaccination. Some people have not showed any side effects, everyone responds differently to the vaccine. It is normal if not having any side effects.
Q)12) How many dose and schedule of Sputnik V Vaccine?
The Sputnik V Vaccine (Gam-COVID-Vac) based on two different extremely safe human adenoviruses as vectors - Adenovirus 26 (Ad26) and Adenovirus 5 (Ad5). Human adenoviruses based medicines have been used widely for more than 50 years.
The Sputnik V - the two-dose schedule vaccine has been administered to the people who are 18 years of age and above. The second dose should be taken after 21 days / 3 weeks gap of the first dose.
Is Sputnik v experimentally administered to any one in the age group of 18 years in India? Will Drug Control Authority of India give clearance to this vaccine without proper trial? Also Serum India cannot keep pace with the requirement of Covishield in India, how do you expect they will be able to produce this large amount of Sputnik V vaccine in time?
ReplyDeleteTill now ,we have not recieved any data,where it is confirmed that Sputnik V can be administered to the age group of18 or below 18,but I will definitelty write a post about Sputnik light which is under experiment ,which is compilled of one single shot /dose and can administered above 60n years of age group.
DeleteIndian population is the 2nd highest poupulation in the entire Worls,so I think Serum India will be delayed but slowly and surely keep their promises to make 139*2 millions of doses of Covisheild as well as Sputnik V too.
Thanks for detailed explanation. I'm getting information much more than my requirements reading this information... excellent and complete work I must admit
ReplyDeleteThanks a lot for this statement, will try to give more info regarding this in future, please stay tuned with our page and blog.Thank you again.
DeleteVery good quality informative writing. Carry on.
ReplyDeleteAbhishek
Thank you so much Abhishek, all of your mental support make me strong to gather more useful information to write another article.
Delete